Журнал «Здоровье ребенка» Том 17, №5, 2022
Вернуться к номеру
Функціонально-біохімічна характеристика м’язової системи в дітей із цукровим діабетом 1-го типу
Авторы: G. Lezhenko (1), O. Pashkova (1), K. Samoylyk (1), A. Brutman (2)
(1) — Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
(2) — National University “Zaporizhzhia Polytechnic”, Zaporizhzhia, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
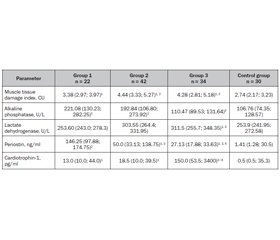
Мета: визначити можливі маркери ураження скелетних м’язів у дітей, хворих на цукровий діабет 1-го типу (ЦД1), та їх зв’язок з особливостями перебігу захворювання. Матеріали та методи. Групу спостереження становили 98 дітей із цукровим діабетом 1-го типу: 1-ша група включала 22 дитини без порушень з боку м’язової системи; 2-га — 42 пацієнти з динапенією; 3-тя — 34 дитини з діабетичною міопатією. Контрольна група — 30 умовно здорових дітей. Усім пацієнтам було проведено дослідження статичної витривалості скелетних м’язів, визначення рівня креатинкінази, аспартатамінотрансферази, лужної фосфатази, лактатдегідрогенази, періостину та кардіотрофіну-1 у сироватці крові. Результати. Проведене дослідження показало, що в дітей, хворих на ЦД1, незалежно від структурно-функціонального стану м’язової системи, спостерігаються ознаки пошкодження скелетних м’язів, що були максимально вираженими при діабетичній міопатії та прогресували при погіршенні глікемічного контролю. Указані порушення відбувалися на тлі змін активності лужної фосфатази, найбільші показники якої спостерігалися в 1-й групі, у той же час у пацієнтів 3-ї групи її вміст у сироватці крові відповідав значенням контрольної групи. Одночасно при розвитку діабетичної міопатії в дітей, хворих на ЦД1, активність лактатдегідрогенази зростала в 1,2 раза (p < 0,01) та кардіотрофіну-1 — у 300 разів (p < 0,01) порівняно з аналогічними показниками контрольної групи. Уміст періостину був підвищеним у всіх групах пацієнтів, хворих на ЦД1. Його значення були максимальними в 1-й групі пацієнтів, перевищуючи показники контрольної групи в 103 рази (p < 0,01). При погіршенні стану скелетної мускулатури рівень періостину в сироватці крові поступово знижувався, але при динапенії залишався вищим за його значення в групі контролю в 35,5 раза (p < 0,05), а при діабетичній міопатії — у 19,2 раза (p < 0,05). Висновки. Перебіг ЦД1 у дітей супроводжується ураженням скелетних м’язів, першою клінічною ознакою якого є зниження статичної витривалості скелетних м’язів, що відбувається на тлі погіршення перебігу захворювання. Біохімічними маркерами ураження скелетних м’язів у дітей, хворих на ЦД1, є лужна фосфатаза, лактатдегідрогеназа, періостін та кардіотрофін-1. Загальною рисою змін означених показників є їх зростання, однак кожному клінічному стану скелетної мускулатури відповідає своя конфігурація змін вказаних маркерів.
Background. The purpose of the study was to determine possible markers of skeletal muscle damage in children with type 1 diabetes mellitus (T1DM) and their relationship with the features of disease course. Materials and methods. The observation group consisted of 98 children with type 1 diabetes mellitus: the first group included 22 people without disorders of the muscular system; the second — 42 patients with dynapenia; the third — 34 children with diabetic myopathy. Control group — 30 relatively healthy children. Assessment of the static endurance of skeletal muscles, determination of the level of creatine kinase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, periostin and cardiotrophin-1 in blood serum were performed in all patients. Results. The conducted studies demonstrate that children with diabetes, regardless of the structural and functional state of their muscular system, have signs of skeletal muscle damage, which were most expressed in diabetic myopathy and progressed with maximal deterioration of glycemic control. It was found that the highest content of alkaline phosphatase was characteristic of children from group 1, while in patients with diabetic myopathy its serum content was not statistically different from that of controls. These disorders occurred against the background of changes in alkaline phosphatase activity, the level of which was highest in children from group 1, while in patients with diabetic myopathy, its serum content was not statistically different from that of controls. At the same time, during the course of diabetic myopathy in children with T1DM, there was an increase in lactate dehydrogenase activity by 1.2 times (p < 0.01) and cardiotrophin-1 by 300 times (p < 0.01) compared to the corresponding indicator of the control group. Serum periostin level was increased in all patients with T1DM. Its maximum values were determined in group 1, whose periostin concentration exceeded control indicators by 103 times (p < 0.01). With deterioration of skeletal muscle state, there was a gradual decrease in periostin serum level, but in patients with dynapenia, it was 35.5 times higher than in the control group (p < 0.05) and 19.2 times higher in those with diabetic myopathy (p < 0.05). Conclusions. The course of type 1 diabetes in children is accompanied by skeletal muscle damage, the first clinical sign of which is a decrease in the static muscle endurance against the background of worsening disease course. Alkaline phosphatase, lactate dehydrogenase, periostin, and cardiotrophin-1 are biochemical markers of skeletal muscle damage in children with type 1 diabetes. A common feature of the changes in the specified indicators is their increase; however, each clinical condition of the skeletal muscles corresponds to its own configuration of changes in the abovementioned markers.
діти; цукровий діабет 1-го типу; діабетична міопатія; маркери ураження скелетних м’язів
children; type 1 diabetes; diabetic myopathy; markers of skeletal muscle damage
Introduction
Materials and methods
Results
Discussion
Conclusions
- Daou H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2020. 318(2). R296-R310. doi: 10.1152/ajpregu.00147.2019.
- Monaco C.M., Gingrich M.A., Hawke T.J. Considering type 1 diabetes as a form of accelerated muscle aging. Exercise and Sport Sciences Reviews. 2019. 47(2). 98-107. doi: 10.1249/JES.0000000000000184.
- Monaco C.M., Perry C.G., Hawke T.J. Diabetic myopathy: current molecular understanding of this novel neuromuscular disorder. Current Opinion in Neurology. 2017. 30(5). 545-552. doi: 10.1097/WCO.0000000000000479.
- Chudova N.I. Early diagnosis, prediction and objectives of approaches to the prevention of muscular system disorders in children suffering from diabetes mellitu. PhD Thesis. Zaporizhzhia State Medical University of the Ministry of Health of Ukraine. Zaporizhzhia, 2022. Available from: https://zsmu.edu.ua/upload/updisert/dfpediatr/12022022_dis_chudova.pdf.
- Zhurakivska O.Y., Koshkin O.Y., Tkachuk Y.L., Knyazevych-Chorna T.V., Rudyak O.M. Age characteristics of morphogenesis of diabetic myopathies. Problems of Endocrine Pathology. 2020. 74(4). 115-123. doi: 10.21856/j-PEP.2020.4.15.
- Coleman S.K., Rebalka I.A., D’Souza D.M., Hawke T.J. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World Journal of Diabetes. 2015. 6(17). 1323. doi: 10.4239/wjd.v6.i17.1323.
- Dimitriadis G.D., Maratou E., Kountouri A., Board M., Lambadiari V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients. 2021. 13(1). 159. doi: 10.3390/nu13010159.
- Type 1 Diabetes Statistics. Available from: https://beyondtype1.org/type-1-diabetes-statistics/.
- Travis C., Srivastava P.S., Hawke T.J., Kalaitzoglou E. Diabetic bone disease and diabetic myopathy: manifestations of the impaired muscle-bone unit in type 1 diabetes. Journal of Diabetes Research. 2022. 2022. doi: 10.1155/2022/2650342.
- Perandini L.A., Chimin P., Lutkemeyer D. da S., Câmara N.O.S. Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: can physical exercise restore the satellite cell niche? The FEBS Journal. 2018. 285(11). 1973-1984. doi: 10.1111/febs.14417.
- Tuttle C.S., Thang L.A., Maier A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Research Reviews. 2020. 64. 101185. doi: 10.1016/j.arr.2020.101185.
- Haberecht-Müller S., Krüger E., Fielitz J. Out of control: the role of the ubiquitin proteasome system in skeletal muscle during inflammation. Biomolecules. 2021. 11(9). 1327. doi: 10.3390/biom11091327.
- Jurisic-Erzen D., Starcevic-Klasan G., Ivanac D., Peharec S., Girotto D., Jerkovic R. The effects of alpha-lipoic acid on diabetic myopathy. Journal of Endocrinological Investigation. 2018. 41(2). 203-209. doi: 10.1007/s40618-017-0720-0.
- Monaco C.M., Hughes M.C., Ramos S.V., Varah N.E., Lamberz C., Rahman F. A., Perry C.G. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia. 2018. 61(6). 1411-1423. doi: 10.1007/s00125-018-4602-6.
- Turner M.C., Player D.J., Martin N.R., Akam E.C., Lewis M.P. The effect of chronic high insulin exposure upon metabolic and myogenic markers in C2C12 skeletal muscle cells and myotubes. Journal of Cellular Biochemistry. 2018. 119(7). 5686-5695. doi: 10.1002/jcb.26748.s.
- Ahmad S.S., Ahmad K., Lee E.J., Lee Y.H., Choi I. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells. 2020. 9(8). 1773. doi: 10.3390/cells9081773.
- Torrente Y., Bella P., Tripodi L., Villa C., Farini A. Role of insulin-like growth factor receptor 2 across muscle homeostasis: Implications for treating muscular dystrophy. Cells. 2020. 9(2). 441. doi: 10.3390/cells9020441.
- Jin B., Zhang L., Wang X., Jin D. Research on orientation of basic fibroblast growth factor with magnetic nanoparticles (MNPs) on regeneration and recovery of rats’ dampened skeletal muscle and expressed level of matrix metalloproteinase. Journal of Biomedical Nanotechnology. 2022. 18(2). 557-564. doi: 10.1166/jbn.2022.3260.
- Ismaeel A., Kim J.S., Kirk J.S., Smith R.S., Bohannon W.T., Koutakis P. Role of transforming growth factor-β in skeletal muscle fibrosis: a review. International Journal of Molecular Sciences. 2019. 20(10). 2446. doi: 10.3390/ijms20102446.
- Peters A.M., Snelling H.L.R., Glass D.M., Bird N.J. Estimation of lean body mass in children. Survey of Anesthesiology. 2012. 56(1). 26-27. doi: 10.1097/01.SA.0000410700.55371.0f.
- Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. American Journal of Physiology-Renal Physiology. 1984. 247(4). F632-F636. doi: 10.1152/ajprenal.1984.247.4.F632.
- Janssen I., Heymsfield S.B., Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002. 50(5). 889-896. doi: 10.1046/j.1532-5415.2002.50216.x.
- Deurenberg P., Weststrate J.A., Seidell J.C. Body mass index as a measure of body fatness: age -and sex-specific prediction formulas. British Journal of Nutrition. 1991. 65(2). 105-114. doi: 10.1079/bjn19910073.
- Akay A.F., Gedik A., Tutus A., Şahin H., Bircan M.K. Body mass index, body fat percentage, and the effect of body fat mass on SWL success. International Urology and Nephrology. 2007. 39(3). 727-730. doi: 10.1007/s11255-006-9133-2.
- Lee J.H., Cho A.R., Lee Y.J. Relationship between serum alkaline phosphatase and low muscle mass index among Korean adults: a nationwide population-based study. Biomolecules. 2021. 11(6). 842. doi: 10.3390/biom11060842.
- Young A., Oldford C., Mailloux R.J. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biology. 2020. 28. 101339. doi: 10.1016/j.redox.2019.101339.
- Ito N., Miyagoe-Suzuki Y., Takeda S.I., Kudo A. Periostin is required for the maintenance of muscle fibers during muscle regeneration. International Journal of Molecular Sciences. 2021. 22(7). 3627. doi: 10.3390/ijms22073627.
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology. 2014. 6(10). a016295. doi: 10.1101/cshperspect.a016295.
- Özdemir C., Akpulat U., Sharafi P., Yıldız Y., Onbaşılar İ., Kocaefe Ç. Periostin is temporally expressed as an extracellular matrix component in skeletal muscle regeneration and differentiation. Gene. 2014. 553. 130-139. doi: 10.1016/j.gene.2014.10.014.
- Raskaliei T.Y., Raskaliei V.B., Shobat L.B., Gavriliuk-Skiba G.O. Histochemical study of skeletal muscles in experimental spinal cord blunt injury. Morphologia. 2016. 10(3). 243-247. doi: 10.26641/1997-9665.2016.3.243-247.
- Pan W., Miao L., Lin Y., Huang X., Ge X., Moosa S. L., Pan L. Regulation mechanism of oxidative stress induced by high glucose through PI3K/Akt/Nrf2 pathway in juvenile blunt snout bream (Megalobrama amblycephala). Fish & Shellfish Immunology. 2017. 70. 66-75. doi: 10.1016/j.fsi.2017.09.005.
- Arnò B., Galli F., Roostalu U., Aldeiri B.M., Miyake T., Albertini A., Cossu G. TNAP limits TGF-β-dependent cardiac and skeletal muscle fibrosis by inactivating the SMAD2/3 transcription factors. Journal of Cell Science. 2019. 132(15). jcs234948. doi: 10.1242/jcs.234948.
- Zhang Z., Nam H.K., Crouch S., Hatch N.E. Tissue nonspecific alkaline phosphatase function in bone and muscle progenitor cells: Control of mitochondrial respiration and ATP production. International Journal of Molecular Sciences. 2021. 22(3). 1140. doi: 10.3390/ijms22031140.
- Ohlebusch B., Borst A., Frankenbach T., Klopocki E., Jakob F., Liedtke D., Graser S. Investigation of alpl expression and Tnap-activity in zebrafish implies conserved functions during skeletal and neuronal development. Scientific Reports. 2020. 10(1). 1-16. doi: 10.1038/s41598-020-70152-5.
- Liedtke D., Hofmann C., Jakob F., Klopocki E., Graser S. Tissue-nonspecific alkaline phosphatase — a gatekeeper of physiological conditions in health and a modulator of biological environments in disease. Biomolecules. 2020. 10(12). 1648. doi: 10.3390/biom10121648.
- Pan Y., Dong Y., Hou W., Ji Z., Zhi K., Yin Z., Chen Y. Effects of PEMF on microcirculation and angiogenesis in a model of acute hindlimb ischemia in diabetic rats. Bioelectromagnetics. 2013. 34(3). 180-188. doi: 10.1002/bem.21755.
- Pashkova O., Chudova N. The role of perifheral circulation disorders in the development of diabetic myopathy in children with diabetes mellitus. Actual Problems of Modern Medicine. 2021. (8). 69-77. doi: 10.26565/2617-409X-2021-8-07.
- Lemire J., Auger C., Mailloux R., Appanna V.D. Mitochondrial lactate metabolism is involved in antioxidative defense in human astrocytoma cells. Journal of Neuroscience Research. 2014. 92(4). 464-475. doi: 10.1002/jnr.23338.
- Field S., Uyttenhove C., Stroobant V., Cheou P., Donckers D., Coutelier J.P., Jat P.S. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. International Journal of Cancer. 2016. 138(8). 1959-1970. doi: 10.1002/ijc.29946.
- Szyszka M., Skrzypczyk P., Stelmaszczyk-Emmel A., Pańczyk-Tomaszewska M. Serum Periostin as a potential biomarker in pediatric patients with primary hypertension. Journal of Clinical Medicine. 2021. 10(10). 2138. doi: 10.3390/jcm10102138.
- Luo Y., Qu H., Wang H., Wei H., Wu J., Duan Y., Deng H. Plasma periostin levels are increased in Chinese subjects with obesity and type 2 diabetes and are positively correlated with glucose and lipid parameters. Mediators of Inflammation. 2016. 2016. 6423637. doi: 10.1155/2016/6423637.
- Miyake T., Alli N.S., Aziz A., Knudson J., Fernando P., Megeney L.A., McDermott J.C. Cardiotrophin-1 maintains the undifferentiated state in skeletal myoblasts. Journal of Biological Chemistry. 2009. 284(29). 19679-19693. doi: 10.1074/jbc.M109.017319.
- Alli N.S. Role and regulation of FRA-2 during skeletal muscle development. Doctoral Dissertation, York University. Toronto, 2014.
- Gamella-Pozuelo L., Fuentes-Calvo I., Gomez-Marcos M.A., Recio-Rodriguez J.I., Agudo-Conde C., Fernández-Martín J.L., Martínez-Salgado C. Plasma cardiotrophin-1 as a marker of hypertension and diabetes-induced target organ damage and cardiovascular risk. Medicine. 2015. 94(30). doi: 10.1097/MD.0000000000001218.
- Escoté X., Gómez-Zorita S., López-Yoldi M., Milton-Laskibar I., Fernández-Quintela A., Martínez J.A., Portillo M.P. Role of omentin, vaspin, cardiotrophin-1, TWEAK and NOV/CCN3 in obesity and diabetes development. International Journal of Molecular Sciences. 2017. 18(8). 1770. doi: 10.3390/ijms18081770.
- Moreno-Aliaga M.J., Pérez-Echarri N., Marcos-Gómez B., Larequi E., Gil-Bea F.J., Viollet B., Bustos M. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metabolism. 2011. 14(2). 242-253. doi: 10.1016/j.cmet.2011.05.013.

/39.jpg)